Regenerative medicine is a relatively young field in the treatment of various conditions. Several novel applications of various types of stem cells have been tried and applied in various aspects of urology. Some published articles provide promising early results.
There are two main sources of stem cells. These are embryonic stem cells (ESCs) and adult-derived stem cells (ADSC). The latter has many sources including bone marrow stem cells (BMSCs), skeletal-muscle-derived stem cells (SkMSCs), adipose-tissue-derived stem cells (ADSCs), and arguably, amniotic-fluid-derived stem cells (AFSCs).
Stem cell use in urology:
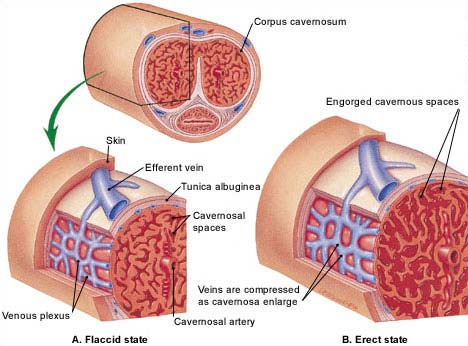
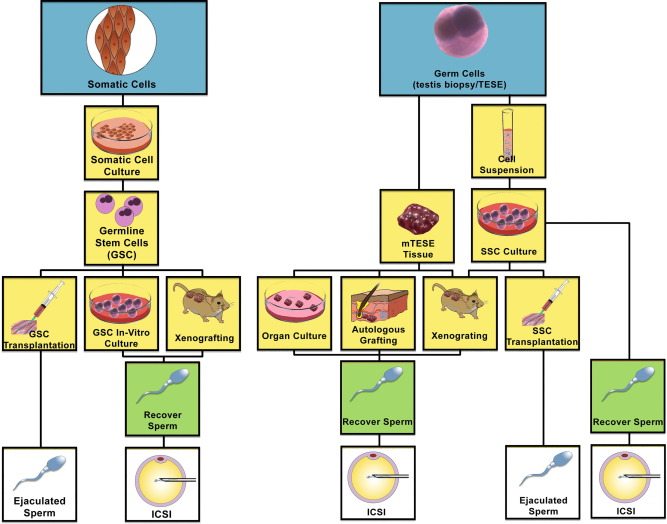
BMSCs, SkMSCs, and AFSCs have been used for bladder augmentation and detrusor regeneration in animals. SkMSCs are the only stem cells to have been successfully tested in humans, for the treatment of stress urinary incontinence. ESCs, BMSCs, and SkMSCs have been shown to improve erectile function in animal models. Both ESCs and BMSCs can be differentiated into sperm and, remarkably, the ESC-derived sperms have generated offspring in mice.
Adipose-derived stem cells:
ADSC research is a relatively young field, and these cells are largely unstudied in urology. However, as a result of their high differentiation potential and ease of isolation, ADSCs represent an exciting resource for tissue engineering and regenerative medicine within and beyond urology.
Male urethral stricture disease:
This is one of the common urological conditions mainly affecting young men, and occasionally older men. The main causes are inflammatory from recurrent urethritis and urethral injury, whether from an accident or iatrogenic.
Until recently, the treatment of urethral stricture is mainly surgical, starting from endoscopic dilatation or urethrotomy, both have a high risk of recurrence, starting from 50% with increasing failure of cure in recurrent ones. Several attempts have been made to combat this problem. Many use intermittent dilatation, that though keeps the uretha patent, it induces more inflammatory reactions from repetitive trauma. The other way is major surgery, called urethroplasty, with a higher success rate but comes at a higher price of complications list (such as infection, bleeding, penile curvature, sexual dysfunction, and of course, recurrence of the stricture).
The main reason for the failure of urethral dilatations is the inflammatory reaction generated by the procedure, with resulting fibrosis.
This where stem cell therapy comes into place. Stem cells provide an alternative way of healing. Once deployed in the area of injury, they differentiate into the local functional cells (in this case the urothelium and possibly, in dense strictures, spongial tissue). This has the potential to prevent the usual inflammatory process that follows trauma, with potentially less scarring.
Hypothesis:
Stem cells, once applied to the area of urethra dilatation/urethrotomy, in the same operative setting, might increase the chance of success of the operation, obviating the need for repetitive endoscopic dilatations and even urethroplasty.
Study proposal and methods:
A prospective randomized double blinded study is proposed, aiming at recruiting 40 participants;
- Group I: n=20 who would receive the conventional urethral dilatation/optical urethrotomy. In addition, they would receive ADSC.
- Group II: n=20 would receive the conventional urethral dilatation/optical urethrotomy.
Procedure (Group I):
Under general anaesthesia (whilst having the urethral dilatation). ADSC will be harvested by liposuction from the abdominal fat using micro-cannula and two tiny abdominal wall incisions. This fat would be then treated for mechanical extraction of the stem cells using purposely made nano blades. The process would take an extra half-an-hour to prepare. The resultant fluid containing stem cells would be then injected via the cystoscope into the area of the urethral dilatation/urethrotomy.
Post-operative care:
Both groups will have the same post-operative protocol; mainly having a urethral catheter, that will remain in situ from several hours up to 5 days depending on the density of the urethral stricture.
Follow up:
Anonymized and coded participants in both groups will have their first post-operative follow-up with an independent and “blinded” clinician, in two weeks, one month, three months, six months, and one year respectively. The follow-up will entail clinical assessment along with UroFlow rate measurement.
Results:
Once analyzed, the results will be written, presented at conferences, and will be published.
Summary:
The future of medicine ( and I dare to say that the possible demise of surgery!) lies in the development and application of stem cells to treat and possibly cure patients with various conditions.
However, as exciting as it seems, this application is quite novel, at least in many medical and surgical specialities, and it’s the application will have to be carried out within a reach, ethics, and Governance framework.
Once properly applied, the prospects are endless