Infertility is a common problem affecting young adults. At least one and six couples would be suffering from this condition. It is the inability to conceive after one year of unprotected intercourse. While traditionally fingers are always pointing towards the female partner, men are equally responsible for inFertility with 50% being caused by the male factor.
Of course, there are other factors causing infertility despite Both partners having normal parameters. When it comes to male factor infertility, there are several causes such as infections, trauma exposure to toxins, or congenital problems. Many of these factors are easily treatable, however, some might require an intervention to facilitate conception (Assisted fertilization). While this started fairly recently in the 80s with limited success, With the advancement of technology and medical sciences, success rates of dramatically increased.
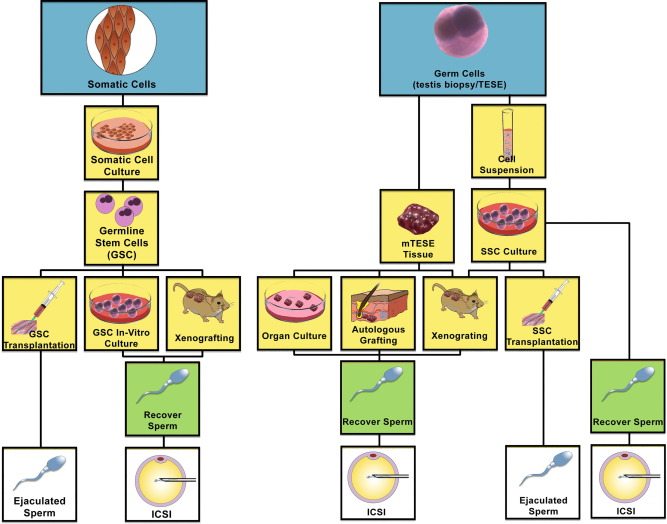
Sperm harvest, though challenging, is becoming more feasible with the improvement in technology. This includes Microscopic testicular sperm extraction, Epididymal sperm aspiration, etc. The application of regenerative medicine in many aspects of healthcare is becoming more popular, however, when treating infertility, this remains to be experimental. Watch the space!
Dr. Ali Thwaini is one of the urologists and provides male infertility treatment in Dubai